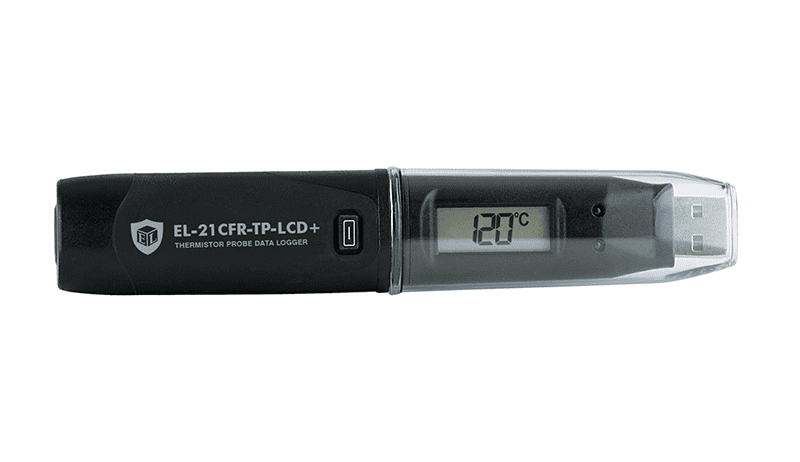
This 21CFR Part 11 compliant standalone data logger records and displays over 32,000 temperature readings from the supplied high accuracy thermistor probe. The data logger features an LCD and push-button which allow the user to cycle through the most recent, highest and lowest stored readings.
Why buy a 21CFR logger?
The 21 CFR Part 11 regulations issued by the US Food and Drug Administration (FDA) give the criteria under which electronic records can be considered by the FDA as equivalent to paper records. If you need to meet these regulations or have enhanced data security requirements then you should choose one of our 21CFR loggers, otherwise our standard loggers will suffice.
Used in conjunction with Lascar’s 21CFR Part 11 software, the logger enjoys all of the core offerings of the original EasyLog software, but with additional 21CFR characteristics including user permissions, encrypted data which cannot be edited, full audit trail and electronic signatures for all activity.
Easily set up the logger and view downloaded data by plugging the unit into a PC’s USB port and using the free 21CFR Part 11 software provided. Supplied with ½ AA battery and wall mount clip.
This product complies with BS EN 12830:2018 (Temperature recorders for the transport, storage, and distribution of temperature-sensitive goods).
PLEASE NOTE: You can add up to TWO SPARE BATTERIES for every data logger ordered. If you wish to order more than this quantity, please contact your local sales office for special shipping information.