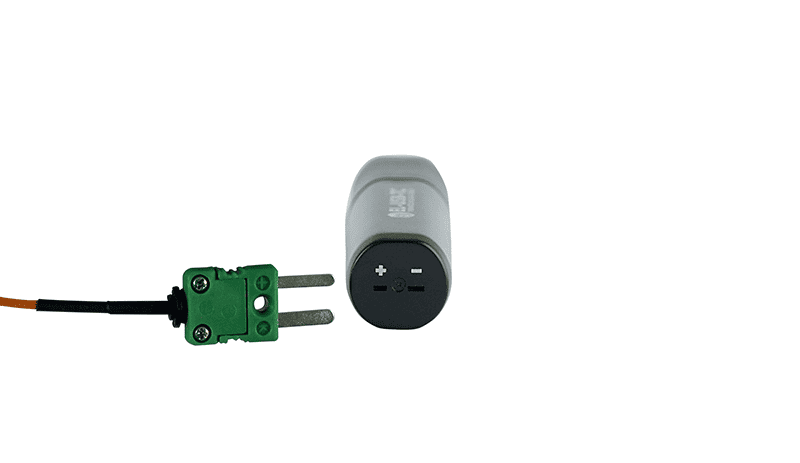
This 21CFR Part 11 compliant ultra-low temperature data logger is designed for monitoring vaccines in cryogenic dry ice storage. The measurement range of the EL-21CFR-ULT is -100 to +100°C (-148 to +212°F) using the probe supplied with the logger. The device can record over 32,000 readings and is simple to set up using the 21CFR Part 11 software, which allows users to set their own high and low alarm thresholds, with alarm hold and delayed start features as well. Flashing lights alert users to any temperature excursions. Just plug the logger into your PC’s USB port to download data which can then be graphed, printed and exported to other applications for detailed analysis.
Why buy a 21CFR logger?
The 21 CFR Part 11 regulations issued by the US Food and Drug Administration (FDA) give the criteria under which electronic records can be considered by the FDA as equivalent to signed paper records. If you need to meet these regulations or have enhanced data security requirements then you should choose one of our 21CFR loggers, otherwise our standard loggers will suffice.
Used in conjunction with Lascar’s 21CFR Part 11 software, the logger enjoys all of the core features of the standard EasyLog software, but with additional 21CFR characteristics including user permissions, encrypted data which cannot be edited, full audit trail and electronic signatures for all activity.
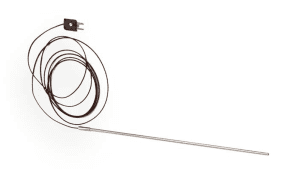
The product is supplied with a ½ AA battery, the probe (300mm probe on a 1m long cable), and a mounting bracket.
This product complies with BS EN 12830:2018 (Temperature recorders for the transport, storage, and distribution of temperature-sensitive goods).
PLEASE NOTE: You can add up to TWO SPARE BATTERIES for every data logger ordered. If you wish to order more than this quantity, please contact your local sales office for special shipping information.